Infection Diagnostic Panels
We carry out research and develop precision diagnostic products based on next-generation sequencing (NGS) technology and supply commercialized products to a number of major hospitals in Korea.
We are striving to lead the precision diagnostics market by providing diagnostic solutions for the entire cancer treatment cycle, from early diagnosis to prognosis/companion diagnosis, and post-operative monitoring and testing.
MTBaccuPanel™
MTBaccuPanel™ is an NGS-based precision diagnostic panel, which allows simultaneous detection of Mycobacterium tuberculosis, identification of nontuberculous mycobacteria species, and detection of mutations associated with drug resistance.
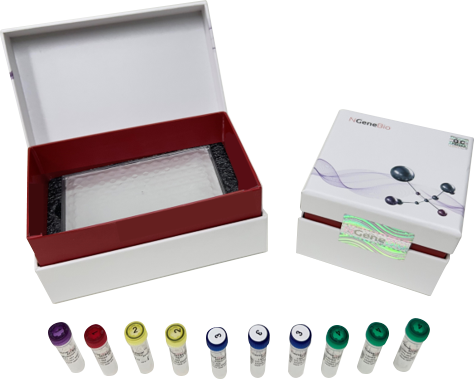
NGS-based Mycobacterium tuberculosis test
MTBaccuPanel™ can simultaneously identify 180 species of non-tuberculous mycobacteria and 18 different types of drugs resistance using NSG. The short turn-around time of 2 days from the test enables appropriate treatment in the early stages of infection.
First NGS-based tuberculosis test in Asia (CE-IVD)
The safety of MTBaccuPanel™ has already been proven by the first CE-IVD in Asia.
- · MTBaccuPanel™ : April 2022

Combination with cloud-based analytics software
MTBaccuPanel™ can be used in combination with automated genome big data analytics software. It is designed to apply and automatically analyze the NGS data using the bioinformatics pipeline and issue a clinical report in a streamlined process.
-

Shortened data analysis time
-

Application of an In-house bioinformatics analysis pipeline
-
Generation of visualized MTB/NTM phylogenetic data
-

Preparation of clinical reports tailored to user needs
-

Provision of drug information using VCF and QC data and drug resistance database
WORKFLOW
-

Genomic DNA
-

Target region
amplification -

Adapter ligation
-

Amplification
-

Next Generation
Sequencing -

NGS Data Analysis &
Create Clinical Reports
Product specification
| MTBaccuPanel | |
|---|---|
| Certification | CE-IVD RUO* |
| Compatible platforms | Illumina / MiSeq*, MiSeq Dx, iSeq 100 |
| Target enrichment | Targeted sequencing / Amplicon method (33-paired primers) |
| Specimen | DNA from human sputum, culture samples, or bronchoalveolar lavage fluid (recommended input DNA: 5ng, LOD: 1ng) |
| Quanity | Up to 24 samples with MiSeq Reagent Kit Micro v2 (300 cycles) Up to 24 samples with iSeq 100 i1 Reagent 2 Kits (300 cycles) |
| Target spp. Identification | Mycobacterium tuberculosis and non-tuberculous mycobacteria (NTM) (around 180 spp.) |
| Anti-tuberculosis drug prediction | INH, RIF, STM, KAN, CAP, AMK, FLQs, EMB, PZA, BDQ, DLM, LNZ etc. (total 18 drugs) |
| Target size | ~31 kb (29 genes, entire genes or specific regions) |
| Turn around time | ~ 5 hrs |
| Analysis solution | Web-based NGB Analysis* (on development) |
*RUO: Reseach Use Only
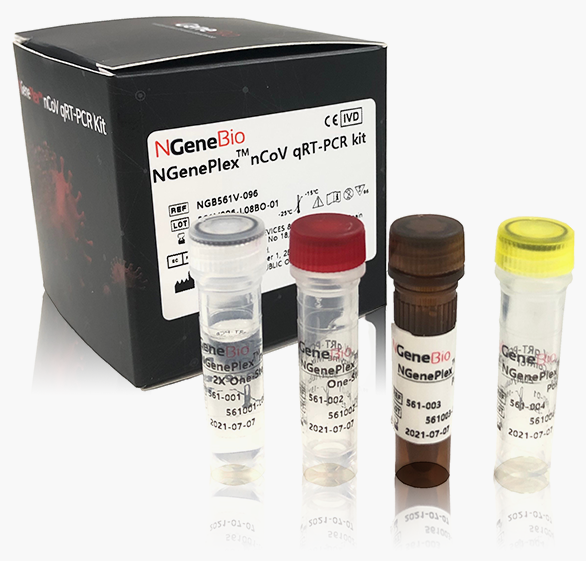
NGenePlex™
nCoV qRT-PCR Kit
NGenePlex™ nCoVqRT-PCR Kit is designed for qualitative detection of SARS-COV-2 (COVID-19) from clinical samples (sputum, oropharyngeal, and/or nasopharyngeal specimens) of suspected patients.
Real-time PCR-based respiratory infection test
It is used for the purpose of detecting SARS-CoV-2 nucleic acids from COVID-19 patients or those suspected of COVID-19 with symptoms such as fever, cough, fatigue, and loss of sense of smell and taste.
Real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis is performed to accurately diagnose COVID-19 infection.
Simultaneous testing for influenza and COVID-19 virus
The kit allows simultaneous detection of influenza (Flu A and B) and COVID-19 (SARS-CoV-2).

CE-IVD certified
In recognition of its accuracy, the kit was approved as a medical device licensed for export by the Ministry of Food and Drug Safety.
Also, in recognition of its safety, it received the CE-IVD certification as an in vitro diagnostic medical device.
- NGenePlex nCoV qRT-PCR Kit · Licensed for export by Korea Ministry of Food and Drug Safety : August 2020 · CE-IVD : July 2020
- NGenePlex nCoV/FluA.B Detection Kit · Licensed for export by Korea Ministry of Food and Drug Safety : June 2022 · CE-IVD : January 2021
WORKFLOW
-
- Step. 1 Sample collection
- Collect specimens from
nasal swab or sputum.
Samples can be stored at
2-8˚C for 1~2 days.

-
- Step. 2 RNA extraction
- Purified RNA is extracted
using a viral RNA extraction kit.

-
- Step. 3 PCR test
- Purified RNA is reverse
transcribed to cDNA and
amplified target genes by qPCR.

-
- Step. 4 Result analysis and report
- Positive SARS-CoV-2
patients cross the threshold line
within 40 cycles (<40 Ct).

Product specification
| NGenePlex™ nCoV/FluA.B Detection Kit | NGenePlex™ nCoV qRT-PCR Kit | |
|---|---|---|
| Certification |
Korean Ministry of Food and Drug Safety (MFDS) license of in-vitro diagnostic device manufacturing (License No. 22-407) CE-IVD |
Korean Ministry of Food and Drug Safety (MFDS) license of in-vitro diagnostic device manufacturing (License No. 20-662) CE-IVD |
| Quantity | 100 tests | 96 tests |
| Reaction time | 80 mins (including ramping time) | |
| Specimen | Human nasopharyngeal (NP) swab, oropharyngeal (OP) swap | |
| Target genes | RdRp and N gene on SARS-CoV-2 NP gene on Influenza A NEP gene on Influenza B |
RdRp and N gene on SARS-CoV-2 |
| Compatible instruments | CFX96™ (Bio-Rad) | CFX96™ (Bio-Rad) ABI 7500 (Applied Biosystems) QuantStudio™3 (Applied Biosystems) LightCycler® 480 (Roche) |
| Fluorometer channel | HEX/VIC (SARS-CoV-2, RdRp/N) FAM (Influenza A, NP) Quasar670 (Influenza B, NEP) Cal Red 610/Texas Red (IC: human RNase P) |
FAM (RdRp) HEX/VIC (N) Cal Red 610/Texas Red (IC: human RNase P) |



